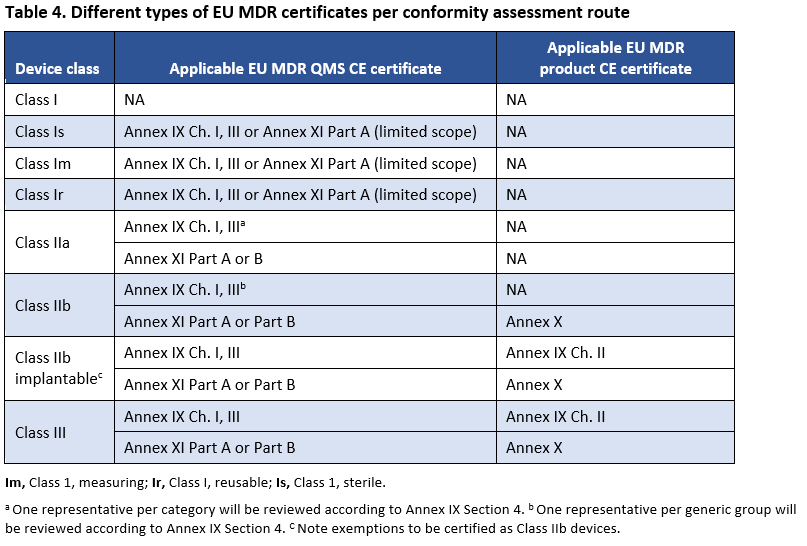
List of conformity assessment bodies (CABs) accredited against the requirements of the eIDAS Regulation | FUTURIUM | European Commission
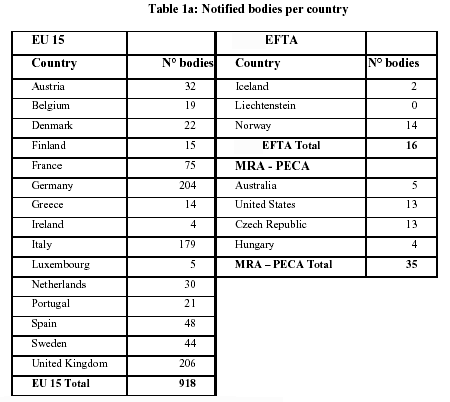
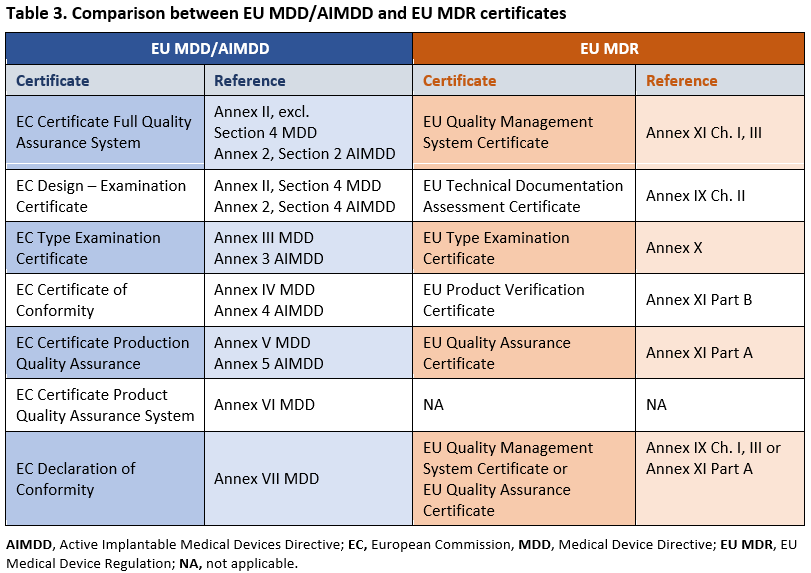
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit